Ochem Which Molecule Is Best Sluable in Water
Submitted by Bill Spencer AMRI. Maleic acid is more soluble because it is more polar with the polar functional groups on the same side of the molecule.
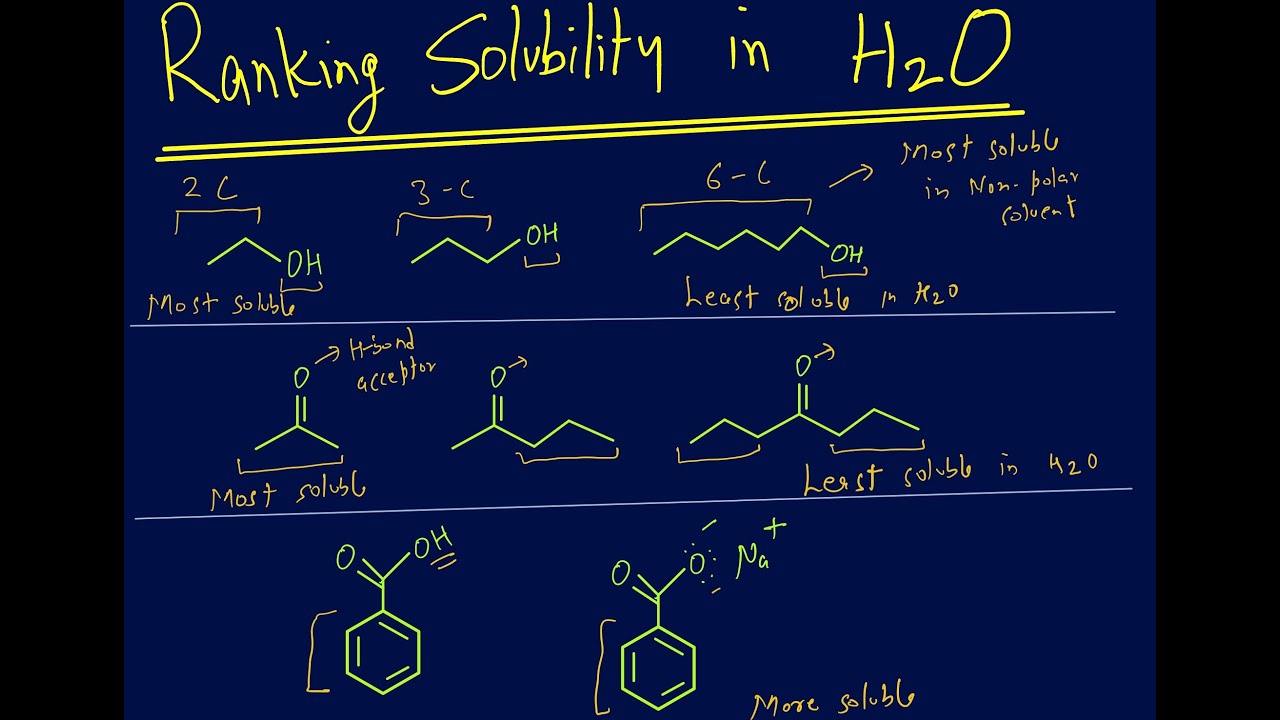
Ranking Solubility Of Organic Compounds In Water Based On Imf Youtube
Alcohols that have a small hydrocarbon chain tend to be very soluble.

. Because water is the biological solvent most biological organic molecules in order to maintain water-solubility contain one or more charged functional groups. The smaller and the more polar the molecule the more soluble it will be in water. Among given compounds ethylene glycol H O C H 2 C H 2 O H is the most soluble in water.
Potassium hydroxide K O H is soluble in water up to 52 52 grams with 48 grams of water. The solubility of ethers decreases with an increase in the number of carbon atoms. As to why sodium benzoate is the sodium salt of benzoic acid and it has an ionic bond between the sodium ion and the organic acid group.
Because 1-heptanol has a much larger non-polar hydrocarbon portion it. What organic molecules are soluble in water. Greater is the number of hydrogen bonds greater is the extent of hydrogen bonding and greater is the solubility in water.
Water is very polar and can surround ions very well. There are many covalent or non-polar compounds that will dissolve in water like sugar carbon dioxide gas and many. This mixture sometimes has the ability to pull aqueous-soluble organics out of the aqueous phase when CH 2 Cl EtOAc Et 2 O etc.
Generally alcohols tend to form hydrogen bonds with water due to the hydroxyl group which is often referred to as a hydrophilic water-loving group. Thus ethers containing up to 3 carbon atoms are soluble in water due to the formation of H-bonds with water molecules. The filtrate is allowed to cool and the mixture is filtered a second time.
The OH- group can form hydrogen bonds with water molecules. Therefore final solution will be acidic aqueous colorless solution. Ethylene glycol has two hydroxy groups both of which form hydrogen bonds with water.
With such property the solubility of alcohol in water is increased. But when those compounds molecular mass increases solubility in water is decreased. Methanol would be more soluble in water.
Ammonium carboxylate phosphate is almost certainly water soluble unless has a vary large nonpolar group in which case it will most likely be soluble in the form of micelles like a soap or detergent. Alcohols are also polar covalent compounds. On the other hand the effect of the degree of substitution on the solubility is less pronounced when the molecular size rise.
Anything with a charged group eg. Water is a polar covalent compound. Maleic acid is about 100 times more soluble in water than fumaric acid.
This why water cannot dissolve oil which is a nonpolar covalent compound. 31 CHCl 3 isopropanol. D which has the maximum number of carbon-hydrogen bonds possible.
In organic chemistry the term unsaturated means a molecule A which contains one or more multiple bonds between carbon atoms. As the hydrocarbyl tail GROWS the solubility of the alcohol in water decreases. Based on its structure which molecule will be the least soluble in hot water.
Here is an excellent organic extraction solvent. From these data it is clear that the internal hydrogen bond is not an impediment to water solubility. These are most often phosphate ammonium or carboxylate all of which are charged when dissolved in an aqueous solution buffered to pH 7.
One of the most common groups is the -OH hydroxyl group. Thus being most soluble in benzene. Benzoyl chloride and water reaction.
Sodium benzoate is soluble in water because it dissolves in water very well to the extent of 60 or so g per 100 g of water depending on the temperature. During the recrystallization of a solid the sample is dissolved in hot solvent then filtered while hot. The sugar molecules themselves are unaffected essentially - instead of all being bound to one another in a crystal they are now floating around in the water.
B which can react by taking up one or more water molecules. Butanol and the higher alcohols exhibit limited water solubility. Propanol is the cut-off point.
C which is formed from many smaller molecules. Because both are alcohols the polar OH group will allow both to participate in dipole-dipole and hydrogen-bonding interactions with water. Benzoic chloride is not soluble in water and form a white precipitate in the water.
According to solubility rules like solutes dissolves in like solvents. Its presence will enable a molecule to be water soluble. Jun 14 2014.
Ethanoic acid is a soluble organic compound. As examples ethanoic acid is soluble in water. And then you can go like attract like to explain why it is soluble in water etc.
E with a specific six-membered ring structure. Generally speaking if the molecule is branched its hydrophobic surface exposed to water is less than in a linear molecule. The same goes for the oxygen which when compared to its neutral atomic state has more electron density and hence a partial negative charge.
A substance is soluble in water when its solid form such as a sugar cube completely dissolves in water to become a sugar solution. Since cells are 70-90 water the degree to which organic molecules interact with water affects their function. Since D has the largest non-polar area it will be most soluble.
Methanol in particular is INSOLUBLE in hexanes. Very non-polar no good ways of interacting with water. For example chloroethane CH3-CH2Cl is polar and has a solubility of 057.
Dimethyl ether C H 3 O C H 3 is soluble in water up to 071 71 grams per liter Wikipedia. Benzoic acid and HCl are given after hydrolysis of benzoyl chloride. Here is my question though I initially looked all of the answers and saw they all had -OH but then I looked at C and saw that it was a symmetrical compound and I assumed that the molecule would be non-polar overall.
Ethers can form hydrogen bonds with water however as water contains the partially positive hydrogen atoms required for H-bonding. Workup for Polar and Water-Soluble Solvents. Benzene C 6 H 6 is soluble in water up to 0188 188 grams per liter.
Alcohols are slightly or relatively soluble in water. Alcohols carboxylic acids carboxylic acid chlorides amines esters are usually soluble in water. The influence of the hydroxyl group confers this water solubility and hexanes insolubility.
The ability to form hydrogen bonds is also a huge advantage especially as size get larger you can see this with many organic molecules add a hydroxyl group -OH to the molecule and solubility increases dramatically. This effect is more pronounced when the number of atoms is bigger. This therefore produces a separation in charges and is basically by definition polar.
Carbon tetrachloride C C l 4 is soluble in water up to 0075 075 grams per liter. The oxygen in the middle can form hydrogen bonds with water molecules.
1 6 Physical Properties Of Organic Compounds Chemistry Libretexts

Why Volume Of Ice Decrease On Melting And Ortho Nitro Phenol Is More Volatile Than Para Nitro Phenol Chemsolve Organic Chemistry Books Ortho Hydrogen Bond

0 Response to "Ochem Which Molecule Is Best Sluable in Water"
Post a Comment